Cast iron
For cast iron the equivalent carbon content (CE) concept is used to understand alloying elements will affect a heat treatments and casting behaviors. The following formulas are used to determine the CE:[4]This CE is then used use to determine if the alloy is hypoeutectic, eutectic, or hypereutectic; for cast irons the eutectic is 4.3% carbon. When casting cast iron this is useful for determining the final grain structure; for example, a hypereutectic cast iron usually has a coarse grain structure and large kish graphite flakes are formed.[7] Also, there is less shrinkage as the CE increases.[6] When heat treating cast iron, various CE samples are tested to empirically determine the correlation between CE and hardness. The following is an example for induction hardened gray irons:[5]
| Composition [%]† | Carbon equivalent‡ | Hardness [HRC] (convert from hardness test) | |||
|---|---|---|---|---|---|
| C | Si | HRC | HR 30 N | Microhardness | |
| 3.13 | 1.50 | 3.63 | 50 | 50 | 61 |
| 3.14 | 1.68 | 3.70 | 49 | 50 | 57 |
| 3.19 | 1.64 | 3.74 | 48 | 50 | 61 |
| 3.34 | 1.59 | 3.87 | 47 | 49 | 58 |
| 3.42 | 1.80 | 4.02 | 46 | 47 | 61 |
| 3.46 | 2.00 | 4.13 | 43 | 45 | 59 |
| 3.52 | 2.14 | 4.23 | 36 | 38 | 61 |
| †Each sample also contained 0.5–0.9 Mn, 0.35–0.55 Ni, 0.08–0.15 Cr, and 0.15–0.30 Mo. ‡Using the CE second equation. | |||||


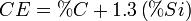

No comments:
Post a Comment